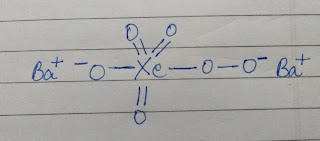There’s a twist in calculation of oxidation state of Xe in BaXeO6 and the twist is that when you will calculate the oxidation state mathematically(by assuming oxidn. state of Xe =y, BA=2, O=-2) you will get 10 as the answer but the correct answer will be +8. Now this is so because oxygen present in BaXeO6 is not present in normal bonding state but rather a per-oxy linkage(image-1) is present due to which two O atoms show -1 oxidation state rather than -2(image-2).
therefore the oxidation state will be calculated as :-
2 + y + 4(-2) + 2(-1) = 0
y + 2 – 10 = 0
y = +8
therefore the oxidation state will be calculated as :-
2 + y + 4(-2) + 2(-1) = 0
y + 2 – 10 = 0
y = +8